Glucotrack Drops the Mic (and Data!): Implantable CGM Trial Results Hitting the ADA Stage
Welcome back to your regularly scheduled Glucotrack, Inc. (GCTK) deep dive! Consider this your definitive guide to their latest SEC filing shenanigans – specifically, the 8-K that dropped on June 4, 2025. We’ve been following GCTK’s story closely (remember their Patient Advisory Board formation and successful first-in-human study announcement last month?), and this new chapter adds some serious intrigue.
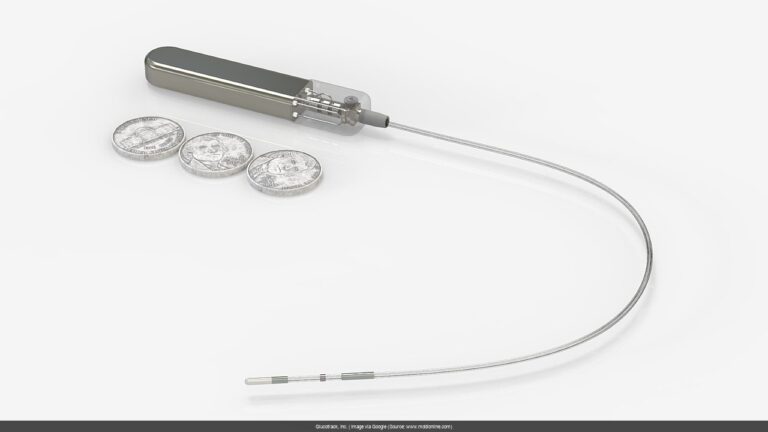
The main 8-K filing itself is pretty straightforward, announcing that GCTK will be presenting data from their first-in-human implantable continuous glucose monitoring (CGM) trial at the American Diabetes Association’s 85th Scientific Sessions (June 20-23, 2025). [[GREEN_FLAG]] Confirmed positive trend! This echoes the good vibes from their previous 8-K on May 20, 2025, which hinted at a successful study. Now, we’re finally getting some concrete details.
GCTK isn’t just showing up; they’re showing *out* with both safety AND performance data. This isn’t just a “we made a thing” presentation; it’s a “our thing works (hopefully)” presentation.
The accompanying EX-99.1 press release (because what’s an 8-K without a press release?) doubles down on this, confirming the presentation format (live presentation *and* a poster – they’re going all in!) and highlighting the potential of their long-term CBGM system to “redefine how diabetes is managed.” [[GREEN_FLAG]] They’re swinging for the fences here, folks. This ambitious goal aligns perfectly with their patient-centric approach emphasized by the formation of the Patient Advisory Board mentioned in the previous EX-99.1 from May 20th.
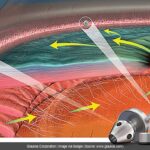
Imagine: up to THREE YEARS of continuous glucose monitoring without wearing anything. That’s the dream GCTK is selling, and now we’re getting closer to seeing if the reality matches the hype.
Of course, the press release also includes the obligatory disclaimer: the CBGM system is still investigational. So, don’t go pre-ordering your implant just yet. But still, the momentum is undeniable.
The Analyst’s Crystal Ball: Glucotrack, Inc. (GCTK) – What Now? (Updated June 04, 2025) 🔮
Sentiment Score from latest documents (this batch only): 90/100 (raw avg: 0.80)
Implication of Current Filings: Positive Momentum Building
Overall Outlook & Forecast
This 8-K is all about anticipation. GCTK is clearly banking on a positive reception at the ADA Scientific Sessions. If their data is compelling, it could be a game-changer. However, remember that “investigational” label – a lot can happen between a successful trial and widespread adoption.
What Would Make Us Yell “To The Moon!” (Go Long) 🚀
- Stellar data presented at the ADA meeting, exceeding expectations.
- Positive feedback from key opinion leaders in the diabetes field.
- Hints at accelerated regulatory pathways for approval.
When We’d Hit The Eject Button (Go Short) 📉
- Disappointing or inconclusive data presented at the ADA meeting.
- Safety concerns raised about the implantable device.
- Signs of regulatory hurdles or delays.
The Mic Drop: So, What’s the Deal with Glucotrack, Inc.’s Latest Paper Trail?
This 8-K is a big deal for Glucotrack. It sets the stage for a potentially pivotal moment at the ADA Scientific Sessions. Will they deliver? We’ll be watching. As always, do your own research (DYOR) and stay tuned for the next chapter in this ongoing SEC saga. Because let’s be honest, reading SEC filings is our kind of party.
Key Questions Answered by This 8-K From Glucotrack, Inc. (GCTK)
-
What is the key takeaway from Glucotrack’s June 4, 2025 8-K filing?
Glucotrack announced they will present data from their first-in-human implantable CGM trial at the American Diabetes Association’s 85th Scientific Sessions.
-
What type of data will Glucotrack present at the ADA Scientific Sessions?
They will present both safety and performance data from their implantable CGM trial.
-
When and where will Glucotrack present this data?
The presentation will take place at the ADA’s 85th Scientific Sessions in Chicago from June 20-23, 2025.
-
How does this 8-K relate to Glucotrack’s previous announcements?
It confirms and builds upon the positive news from the May 20, 2025 8-K, which announced the successful completion of the first-in-human trial.
-
What is the significance of this data presentation for Glucotrack?
It provides a platform for data dissemination and potential validation within the medical community, marking a major milestone for the company.
-
What is the current status of Glucotrack’s CBGM system?
It is considered an investigational device and is limited to investigational use.
-
What is the potential impact of Glucotrack’s CBGM system on diabetes management?
It has the potential to “redefine how diabetes is managed” by offering direct blood glucose measurements without wearables for up to three years, according to the company.
P.S. The SEC saga never ends! As Glucotrack, Inc. files more, this analysis will evolve. Current as of June 04, 2025.