Rocket Pharmaceuticals Stock Plummets: Patient Death in Clinical Trial Triggers FDA Clinical Hold 🚨
Welcome back to the ongoing saga of Rocket Pharmaceuticals, Inc. (RCKT), where we decode the cryptic messages hidden within their SEC filings. Consider this your definitive guide to the latest chapter – and it’s a doozy.
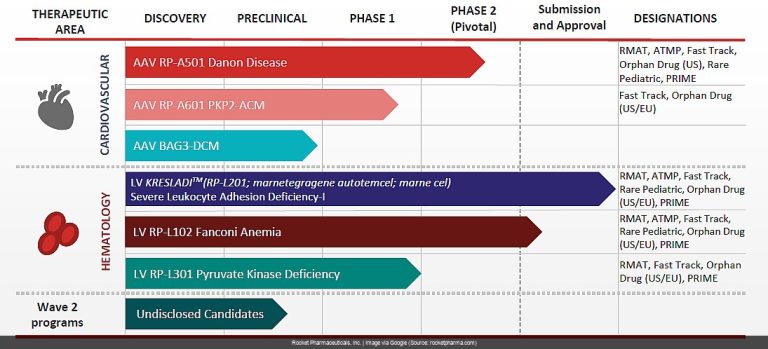
Rocket Pharmaceuticals filed an 8-K on May 27, 2025, and let’s just say it wasn’t filled with good news. The initial filing (8-K) itself was brief, mentioning a clinical hold on their Phase 2 trial of RP-A501 for Danon disease. [[RED_FLAG]] Not ideal, but sometimes these things happen, right? Hold your horses, because the accompanying press release (EXHIBIT 99.1) paints a much grimmer picture.
A patient in the RP-A501 trial died following a serious adverse event related to capillary leak syndrome. Yeah, you read that right. [[RED_FLAG]]
The company is now investigating the cause, with a newly introduced immune suppression agent under scrutiny. [[RED_FLAG]] While the 8-K confirmed their cash runway extends into 2027 (excluding potential voucher sales) – something we’ve discussed previously – this news throws a considerable wrench into their plans. [[RED_FLAG]]
This tragic event raises serious questions about the safety and future of RP-A501.
The Analyst’s Crystal Ball: Rocket Pharmaceuticals, Inc. (RCKT) – What Now? (Updated May 27, 2025) 🔮
Sentiment Score from latest documents (this batch only): 10/100 (raw avg: -0.80)
Implication of Current Filings: Major Negative Impact
Overall Outlook & Forecast
This is a devastating blow for Rocket Pharmaceuticals. The clinical hold and, more importantly, the patient death, casts a long shadow over the future of RP-A501. The investigation into the immune suppression agent is critical, and the outcome will likely determine the fate of this drug candidate. The company’s financial position, while seemingly stable for now, could quickly deteriorate if RP-A501 is permanently shelved.
What Would Make Us Yell “To The Moon!” (Go Long) 🚀
- The investigation exonerates RP-A501 and identifies the immune suppression agent as the sole cause of the SAE, allowing the trial to resume quickly.
- Positive data emerges from other pipeline programs, offsetting the negative impact of the RP-A501 hold.
When We’d Hit The Eject Button (Go Short) 📉
- The investigation implicates RP-A501 in the patient’s death, leading to its discontinuation.
- Further adverse events occur in other trials, suggesting systemic issues with Rocket’s drug development process.
The Mic Drop: So, What’s the Deal with Rocket Pharmaceuticals, Inc.’s Latest Paper Trail?
This 8-K filing from Rocket Pharmaceuticals is more than just a bump in the road; it’s a potential roadblock. The patient death and subsequent clinical hold on RP-A501 are significant setbacks, with far-reaching implications for the company’s future. As always, do your own research (DYOR) and stay tuned for further updates.
Key Questions Answered by This 8-K From Rocket Pharmaceuticals, Inc. (RCKT)
-
Why did the FDA place a clinical hold on Rocket Pharmaceuticals’ Phase 2 trial of RP-A501 for Danon disease?
The FDA placed the trial on hold due to the death of a patient after experiencing a serious adverse event (SAE) related to capillary leak syndrome.
-
What is the company investigating as a potential cause of the serious adverse event?
Rocket Pharmaceuticals is investigating a recently introduced immune suppression agent as a potential contributing factor to the SAE.
-
What is the company’s current financial outlook?
Rocket Pharmaceuticals has $318.2 million in cash, cash equivalents, and investments as of March 31, 2025, and expects this to fund operations into 2027, excluding potential proceeds from the sale of Priority Review Vouchers.
-
What was the specific serious adverse event that led to the patient’s death?
The SAE involved clinical complications related to capillary leak syndrome.
-
What is the next step for the RP-A501 clinical trial?
The trial is currently on hold while the company investigates the SAE and works with the FDA to determine the next steps.
P.S. The SEC saga never ends! As Rocket Pharmaceuticals, Inc. files more, this analysis will evolve. Current as of May 27, 2025.