Savara’s FDA Dreams Go Poof! Molbrevi BLA Refused, Stock Likely to Tank 📉
Welcome back to the Savara Inc. saga, dear readers! Consider this your definitive guide to the latest plot twist. Today, we’re dissecting the company’s 8-K filing from May 27, 2025—a real page-turner, if you’re into regulatory heartbreak. Spoiler alert: it’s not pretty.
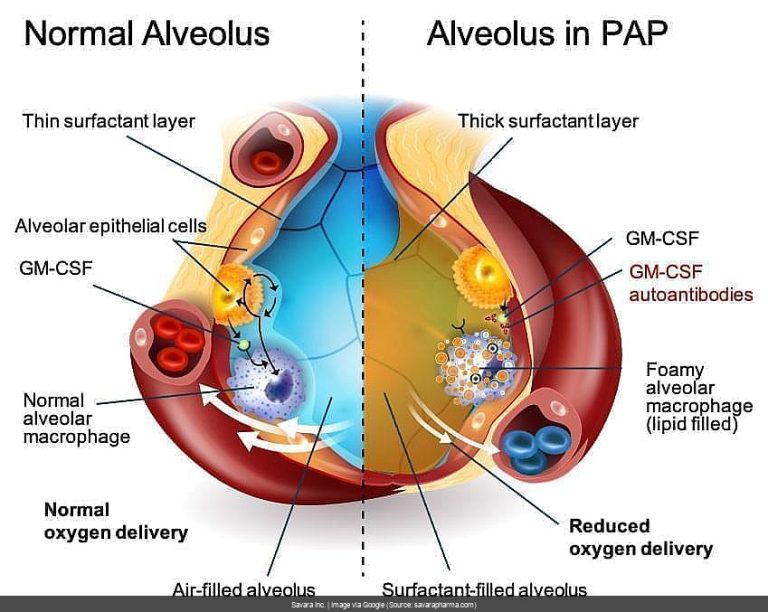
The main 8-K filing itself was brief, like a bad breakup text. It announced that Savara received a Refusal to File (RTF) letter from the FDA regarding their Biologics License Application (BLA) for molgramostim (aka Molbrevi), intended to treat autoimmune pulmonary alveolar proteinosis (aPAP). [[RED_FLAG]] Ouch.
The FDA just served Savara a steaming plate of “No Thanks” on their Molbrevi application. This is not a drill, folks.
But the real dirt is in the EX-99.1 press release, which gives us the gory details. Apparently, the FDA isn’t satisfied with the Chemistry, Manufacturing, and Controls (CMC) data. [[RED_FLAG]] They want more information before they’ll even *consider* filing the application. This pushes the potential resubmission back to Q4 2025. [[RED_FLAG]] Double ouch.
Now, the silver lining (if you can call it that) is that the FDA didn’t flag any safety or efficacy concerns. This at least confirms what we saw in the initial 8-K – no news is sometimes good news in the world of biotech. Savara also mentions they’re working on a redundant supply chain, which, you know, is probably something they should’ve had nailed down before submitting a BLA. Just sayin’.
No safety or efficacy concerns…yet. But this CMC snag is like forgetting your passport on the way to the airport – frustrating and avoidable.
The Analyst’s Crystal Ball: Savara Inc. (SVRA) – What Now? (Updated May 27, 2025) 🔮
Sentiment Score from latest documents (this batch only): 15/100 (raw avg: -0.70)
Implication of Current Filings: Significant Negative Impact
Overall Outlook & Forecast
This RTF is a major blow to Savara. While the lack of safety/efficacy concerns is a small positive, the CMC issues and the resulting delay are substantial setbacks. This adds uncertainty and will likely impact investor confidence.
What Would Make Us Yell “To The Moon!” (Go Long) 🚀
- Savara swiftly addresses the FDA’s CMC concerns and resubmits the BLA ahead of schedule.
- Positive news emerges from other pipeline developments, offsetting the Molbrevi setback.
When We’d Hit The Eject Button (Go Short) 📉
- Further delays or complications arise in addressing the CMC issues.
- Savara experiences a significant cash crunch, hindering their ability to resolve the RTF.
The Mic Drop: So, What’s the Deal with Savara Inc.’s Latest Paper Trail?
This 8-K filing from Savara is a big deal. The FDA’s refusal to file the Molbrevi BLA throws a wrench in the company’s plans and raises serious questions about their preparedness. While there’s still a glimmer of hope, investors should proceed with extreme caution. As always, do your own research (DYOR) before making any investment decisions.
Key Questions Answered by This 8-K From Savara Inc. (SVRA)
-
Why did Savara Inc. receive a Refusal to File letter from the FDA?
The FDA issued the RTF letter because of insufficient Chemistry, Manufacturing, and Controls (CMC) data related to their Molbrevi BLA for aPAP.
-
Did the FDA cite any safety or efficacy concerns regarding Molbrevi?
No, the RTF letter did not mention any safety or efficacy concerns.
-
When does Savara Inc. expect to resubmit the BLA for Molbrevi?
Savara anticipates resubmitting the BLA in the fourth quarter of 2025.
-
What is Savara Inc. doing to address the CMC issues?
Savara is currently generating the requested CMC data and working on establishing a redundant supply chain.
-
What is the overall sentiment surrounding this 8-K filing?
The overall sentiment is negative due to the RTF and the resulting delay in the Molbrevi approval process.
-
Where can I find the official 8-K filing and press release?
P.S. The SEC saga never ends! As Savara Inc. files more, this analysis will evolve. Current as of May 27, 2025.